Drugs advertised as alternatives to Ozempic, Wegovy that pose health risks
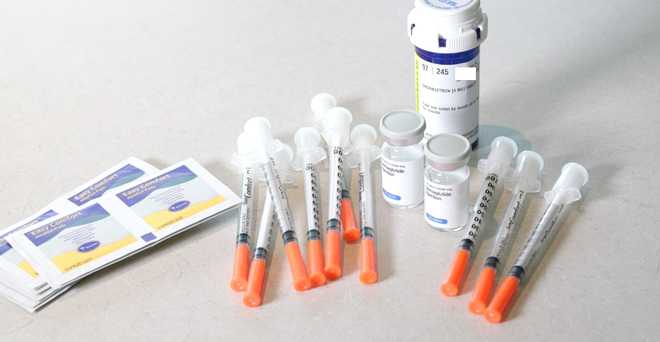
As drugs like Wegovy and Ozempic become more difficult to obtain, 5 Investigates found that some people across Greater Boston who are looking for help with weight loss are turning to potentially dangerous alternatives. But with one of the side effects being weight loss, it started a frenzy among people wanting weight loss help and caused a nationwide shortage. Some people then turned to online telehealth companies or local medspas to get what they thought was Wegovy or Ozempic. Wegovy and Ozempic have been hailed as a major diabetes breakthrough, as the drugs mimic a digestive hormone that can lower blood sugar levels, curb appetite and slow digestion. Dr. Christopher Davidson, a board-certified plastic surgeon, told 5 Investigates that one of his colleagues felt sick after she took what she thought was Ozempic that she bought from a medspa. His colleague did not want to be interviewed, but she gave Davidson permission to tell her story. “She has never called in sick in 10 years. She had to call in sick the day after taking this drug,” said Davidson. “What kind of symptoms did she have?” asked Brittany Johnson of 5 Investigates.”She had nausea, she had gastrointestinal symptoms, she had general malaise, she had loss of appetite, loss of energy,” Davidson replied. “She showed me a picture of Ozempic in her lettuce drawer in her fridge and there were these two unmarked syringes and we started talking, is that really Ozempic at all?” Davidson said. According to Davidson, the drug his colleague had was from a pharmacy in Miami. Compounding pharmacies are specialty pharmacies that provide treatments for individuals who cannot receive traditional medications. When there are drug shortages, the Food and Drug Administration allows designated compounding pharmacies to make versions of the drug that is in high demand. Telehealth companies and medspas across Massachusetts are now using this exemption to claim that the main ingredient in Ozempic and Wegovy — semaglutide — can be mixed and used as a name brand “generic” form. The FDA has issued a warning against the semaglutide complex, stating “Patients should be aware that some products marketed as ‘semaglutide’ may not contain the same active ingredient as FDA-approved semaglutide products,” such as Ozempic and Wegovy, and that they “have not been shown to be safe and effective.” “There are no generics for these drugs,” said Northeastern University Pharmacy Law Professor Kelly Ann Barnes. “Generic drugs are approved similar to the brand-name drugs available on the market through a process with the FDA,” Barnes said. “Compound drugs don’t go through those kinds of standards. And so it’s not a generic.” Barnes cautioned that the sodium semaglutide these pharmacies often use is not the same as the name-brand prescriptions, and that compounding pharmacies are not as well established. to the mass production of drugs on this scale. “How do we know that in these compounded versions, there is as much semaglutide as there is in Ozempic and Wegovy?” asked Johnson. “You don’t know,” Barnes replied. People have reported illnesses such as gastrointestinal ailments, seizures and even hospitalizations. The FDA received 239 adverse event reports related to compounded semaglutide, but the number of records is limited because compounding pharmacies are not required to report adverse events and often do not. Johnson bought semaglutide from two different companies online so she could see firsthand what the process was like. After Johnson submitted her medical history, both companies said she would have a telehealth call and that a doctor would review her medical intake form before finally determining whether she was approved for the drug. Although every website said there would be a telehealth call with a doctor, Johnson never met one-on-one with a doctor. Hours later, Johnson received video messages from board-certified physicians saying she had been approved for semaglutide. 5 Investigators sought the doctors’ credentials and found that one was a dermatologist, while the other was an OBGYN. Barnes said it “definitely raises some concerns” that doctors with these seemingly unrelated specialties were the doctors who approved Johnson for a weight loss. “I think scope of practice is definitely something that needs to be looked at in terms of who is actually prescribing these drugs,” she said. Both of Johnson’s prescriptions included bottles of semaglutide solution, insulin syringes and anti-nausea medication. Barnes said the biggest risk with these drugs is not knowing what you’re taking — so buyer beware. “Just make sure you understand where the drug is coming from, the pharmacy that’s properly licensed, that it’s accredited, and just do your homework so you’re safe,” Barnes said. The company that makes Ozempic, Novo Nordisk, said it has filed 12 lawsuits against compounding and compounding pharmacies and will continue to pursue legal action against companies that claim it contains semaglutide.
As drugs like Wegovy and Ozempic become more difficult to obtain, 5 Investigates found that some people across Greater Boston who are looking for help with weight loss are turning to potentially dangerous alternatives.
But with one of the side effects being weight loss, it caused a frenzy among people wanting help with weight loss and caused a nationwide shortage. Some people then turned to online telehealth companies or local medspas to get what they thought was Wegovy or Ozempic.
Wegovy and Ozempic have been hailed as a breakthrough for diabetes, as the drugs mimic a digestive hormone that can lower blood sugar levels, curb appetite and slow digestion.
Dr. Christopher Davidson, a board-certified plastic surgeon, told 5 Investigates that one of his colleagues felt sick after she took what she thought was Ozempic that she bought from a medspa.
His colleague did not want to be interviewed, but she gave Davidson permission to tell her story.
“She has never called in sick in 10 years. She had to call in sick the day after taking this drug,” Davidson said.
“What kind of symptoms did she have?” asked Brittany Johnson of 5 Investigates.
“She had nausea, she had gastrointestinal symptoms, she had general malaise, she had loss of appetite, loss of energy,” Davidson replied.
“She showed me a picture of Ozempic in her lettuce drawer in her refrigerator and she had these two unmarked syringes and we started talking, is this really Ozempic at all?” Davidson said.
According to Davidson, the drug his colleague had was from a pharmacy in Miami.
Compound pharmacies are specialized pharmacies that provide treatments for individuals who cannot take traditional medications. When there are drug shortages, the Food and Drug Administration allows designated compounding pharmacies to make versions of the drug that is in high demand.
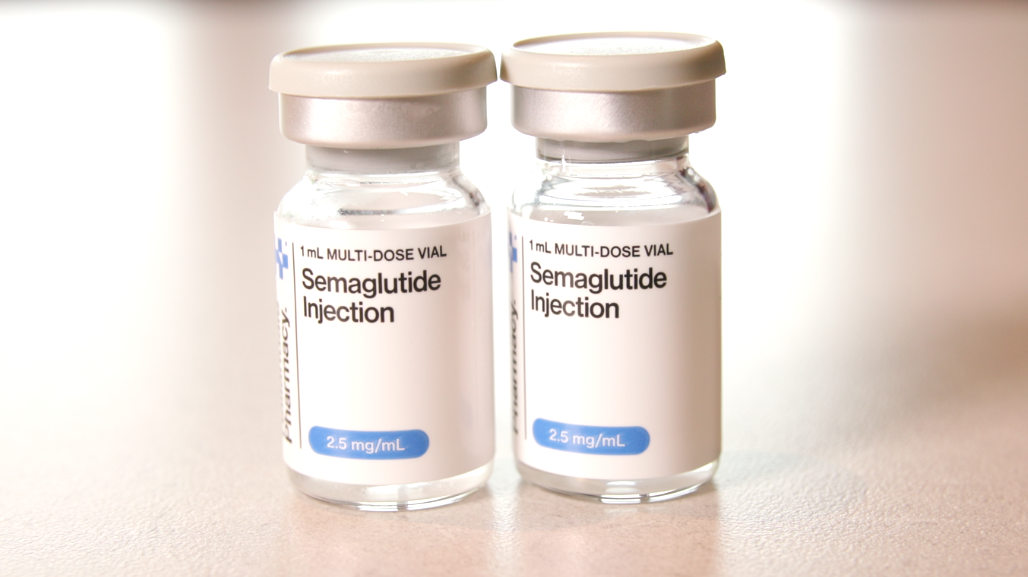
Telehealth companies and medspas across Massachusetts are now using this exemption to claim that the main ingredient in Ozempic and Wegovy — semaglutide — can be mixed and used as a name brand “generic” form.
The FDA has issued a warning against compound semaglutide, saying “Patients should be aware that some products marketed as ‘semaglutide’ may not contain the same active ingredient as FDA-approved semaglutide products,” such as Ozempic and Wegovy , and that they “have not been shown to be safe and effective.”
“There are no generics for these drugs,” said Northeastern University pharmacy law professor Kelly Ann Barnes.
“Generic drugs are approved similar to the brand-name drugs available on the market through a process with the FDA,” Barnes said. “Compound drugs don’t go through those kinds of standards. And so it’s not a generic.”
Barnes cautioned that the sodium semaglutide these pharmacies often use is not the same as the name-brand prescriptions, and that compounding pharmacies are also not designed to mass-produce drugs on this scale.
“How do we know that in these compounded versions, there is as much semaglutide as there is in Ozempic and Wegovy?” asked Johnson.
“You don’t know,” Barnes replied.
People have reported illnesses such as gastrointestinal ailments, seizures and even hospitalizations.
The FDA received 239 reports of adverse events related to compounded semaglutide, but the number of records is limited because compounding pharmacies are not required to report adverse events and often do not.
Johnson bought semaglutide from two different companies online so she could see firsthand what the process was like.
After Johnson submitted her medical history, both companies said she would have a health call and that a doctor would review her medical intake form before finally determining whether she was approved for the drug.
Although every website said there would be a phone call with a doctor, Johnson never met one-on-one with a doctor.
Hours later, Johnson received video messages from board-certified physicians saying she had been approved for semaglutide. 5 Investigators sought the doctors’ credentials and found that one was a dermatologist and the other an OBGYN.
Barnes said it “definitely raises some concerns” that doctors with these seemingly unrelated specialties were the doctors who approved Johnson for a weight-loss drug.
“I think scope of practice is definitely something that needs to be looked at in terms of who is actually prescribing these drugs,” she said.
Both of Johnson’s prescriptions included bottles of semaglutide solution, insulin syringes and anti-nausea medication.
Barnes said the biggest risk with these drugs is not knowing what you’re taking — so buyer beware.
“Just make sure you understand where the drug is coming from, the pharmacy that’s properly licensed, that it’s accredited, and just do your homework so you’re safe,” Barnes said.
The company that makes Ozempic, Novo Nordisk, said it has filed 12 lawsuits against compounding and compounding pharmacies and will continue to pursue legal action against companies that claim it contains semaglutide.
#Drugs #advertised #alternatives #Ozempic #Wegovy #pose #health #risks
Image Source : www.wcvb.com